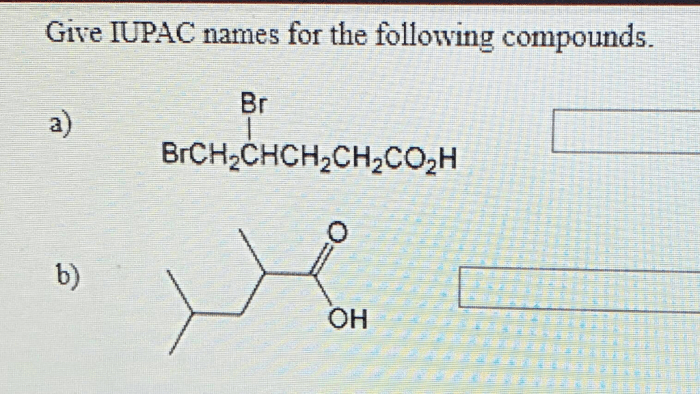
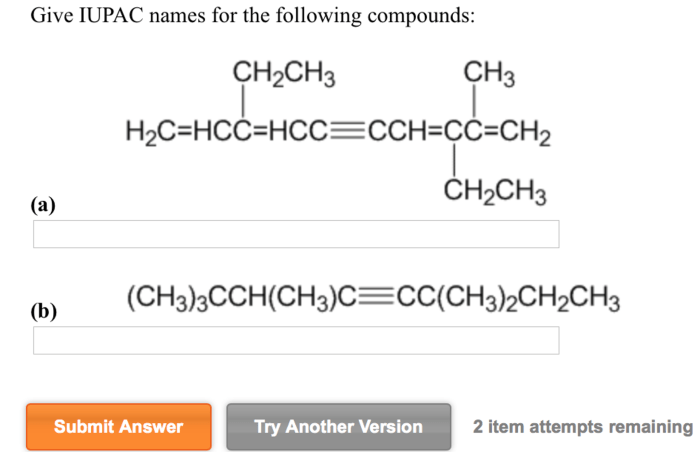
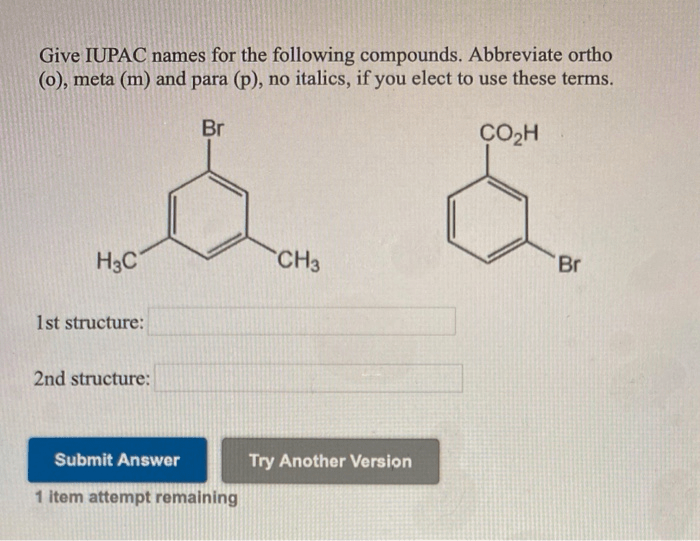
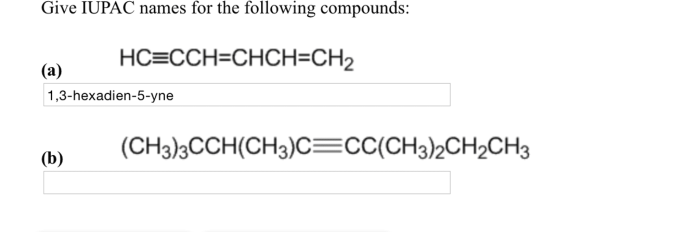
Give the IUPAC names of the following compounds: this seemingly straightforward task lies at the heart of effective scientific communication. The International Union of Pure and Applied Chemistry (IUPAC) has established a systematic nomenclature system to ensure clarity and consistency in naming organic compounds.
Understanding and applying IUPAC rules empowers chemists to accurately convey complex molecular structures, facilitating collaboration, research, and the advancement of chemistry.
This comprehensive guide delves into the intricacies of IUPAC nomenclature, providing a step-by-step approach to naming various classes of organic compounds. From simple alkanes to complex carboxylic acids, we will explore the rules governing the selection of parent chains, prefixes, and suffixes, enabling you to confidently assign IUPAC names to any given compound.
IUPAC Nomenclature
The International Union of Pure and Applied Chemistry (IUPAC) has established a set of rules for naming organic compounds. These rules are essential for clear and consistent communication in the scientific community, as they provide a systematic way to identify and describe organic molecules.
Functional Groups
Organic compounds are classified based on the presence of functional groups, which are specific atoms or groups of atoms that impart characteristic chemical properties. Common functional groups include:
- Alkanes: Contain only carbon and hydrogen atoms, with all carbon atoms bonded by single bonds.
- Alkenes: Contain at least one carbon-carbon double bond.
- Alkynes: Contain at least one carbon-carbon triple bond.
- Alcohols: Contain a hydroxyl group (-OH) bonded to a carbon atom.
- Ethers: Contain an oxygen atom bonded to two carbon atoms.
- Aldehydes: Contain a carbonyl group (-CHO) bonded to a carbon atom.
- Ketones: Contain a carbonyl group (-CO-) bonded to two carbon atoms.
- Carboxylic acids: Contain a carboxyl group (-COOH) bonded to a carbon atom.
Alkanes
Alkanes are named based on the number of carbon atoms in the parent chain. The suffix “-ane” is added to the root of the name, which indicates the number of carbon atoms. For example:
- Methane: CH 4
- Ethane: C 2H 6
- Propane: C 3H 8
- Butane: C 4H 10
Alkenes
Alkenes are named based on the number of carbon atoms in the parent chain and the location of the double bond. The suffix “-ene” is added to the root of the name, which indicates the number of carbon atoms. The location of the double bond is indicated by a number preceding the suffix.
For example:
- Ethene: C 2H 4
- Propene: C 3H 6
- 1-Butene: C 4H 8
- 2-Butene: C 4H 8
Alkynes
Alkynes are named based on the number of carbon atoms in the parent chain and the location of the triple bond. The suffix “-yne” is added to the root of the name, which indicates the number of carbon atoms. The location of the triple bond is indicated by a number preceding the suffix.
For example:
- Ethyne: C 2H 2
- Propyne: C 3H 4
- 1-Butyne: C 4H 6
- 2-Butyne: C 4H 6
Alcohols
Alcohols are named based on the number of carbon atoms in the parent chain and the location of the hydroxyl group. The suffix “-ol” is added to the root of the name, which indicates the number of carbon atoms. The location of the hydroxyl group is indicated by a number preceding the suffix.
For example:
- Methanol: CH 3OH
- Ethanol: C 2H 5OH
- 1-Propanol: C 3H 7OH
- 2-Propanol: C 3H 7OH
Ethers, Give the iupac names of the following compounds
Ethers are named based on the two alkyl groups bonded to the oxygen atom. The names of the alkyl groups are listed in alphabetical order, followed by the word “ether”. For example:
- Dimethyl ether: CH 3OCH 3
- Ethyl methyl ether: CH 3CH 2OCH 3
- Diethyl ether: CH 3CH 2OCH 2CH 3
Aldehydes
Aldehydes are named based on the number of carbon atoms in the parent chain and the location of the carbonyl group. The suffix “-al” is added to the root of the name, which indicates the number of carbon atoms. The location of the carbonyl group is indicated by a number preceding the suffix.
For example:
- Formaldehyde: HCHO
- Acetaldehyde: CH 3CHO
- Propanal: C 3H 6O
- Butanal: C 4H 8O
Ketones
Ketones are named based on the number of carbon atoms in the parent chain and the location of the carbonyl group. The suffix “-one” is added to the root of the name, which indicates the number of carbon atoms. The location of the carbonyl group is indicated by a number preceding the suffix.
For example:
- Acetone: CH 3COCH 3
- 2-Butanone: CH 3CH 2COCH 3
- 3-Pentanone: CH 3CH 2CH 2COCH 3
Carboxylic Acids
Carboxylic acids are named based on the number of carbon atoms in the parent chain and the location of the carboxyl group. The suffix “-oic acid” is added to the root of the name, which indicates the number of carbon atoms.
The location of the carboxyl group is indicated by a number preceding the suffix. For example:
- Formic acid: HCOOH
- Acetic acid: CH 3COOH
- Propionic acid: C 3H 5COOH
- Butyric acid: C 4H 7COOH
Frequently Asked Questions: Give The Iupac Names Of The Following Compounds
What is the importance of IUPAC nomenclature?
IUPAC nomenclature provides a standardized system for naming organic compounds, ensuring clarity and consistency in scientific communication. It enables chemists to accurately describe and identify compounds, regardless of their complexity or origin.
How do I determine the parent chain in an organic compound?
The parent chain is the longest continuous chain of carbon atoms in the compound. It forms the основу for the IUPAC name and determines the prefixes and suffixes used.
What are the rules for naming functional groups?
Functional groups are specific groups of atoms within a molecule that confer characteristic chemical properties. IUPAC nomenclature assigns specific prefixes and suffixes to indicate the presence and location of functional groups.